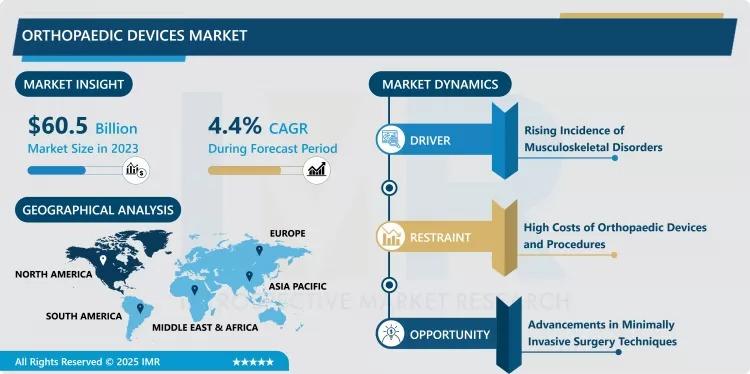
Introspective Market Research (IMR) has released a definitive analysis of the Global Orthopaedic Devices Market, revealing a landscape rapidly being reshaped by digital surgery and patient-specific implant design. The market, valued at USD 60.5 Billion in 2023, is forecast to achieve a valuation of USD 89.14 Billion by the end of 2032, reflecting a robust Compound Annual Growth Rate (CAGR) of 4.4% across the 2024–2032 forecast period.
This sustained growth is predominantly fueled by an alarming global surge in musculoskeletal disorders (MSDs), directly linked to the aging global population and the concurrent rise in obesity, sports injuries, and trauma-related incidents. However, the qualitative growth engine of this market is the dramatic shift from conventional surgical tools to sophisticated, technology-integrated platforms—including robotics, advanced navigation systems, and 3D printing—which are collectively pushing the boundaries of surgical accuracy, implant longevity, and patient recovery speed.
Quick Insights: The Orthopaedic Devices Market Snapshot
- Market Valuation (2023): USD 60.5 Billion
- Projected Market Valuation (2032): USD 89.14 Billion
- Growth Rate (CAGR 2024–2032): 4.4%
- Key Market Driver: The escalating prevalence of chronic musculoskeletal disorders (arthritis, osteoporosis) and increasing sports-related injuries worldwide.
- Leading Segment (Type): Joint Reconstruction Devices (Knee and Hip systems dominate, driven by demand for total joint arthroplasty).
- Leading Segment (End User): Hospitals & Ambulatory Surgery Centers (ASCs) (ASCs, in particular, are gaining traction for less complex, elective procedures).
- Dominant Regional Market: North America (Characterized by high surgical volumes, rapid adoption of expensive robotic systems, and favorable reimbursement).
- Key Opportunity: The convergence of Minimally Invasive Surgery (MIS) techniques with 3D-printed, patient-specific implants.
- Top Industry Players: A concentrated core of global leaders including Zimmer Biomet Holdings, Stryker Corporation, DePuy Synthes, Medtronic plc, and Smith & Nephew, locked in a fierce innovation race.
Orthopaedic Devices Market Revenue Breakdown (2024-2032 Forecast)
| Metric | 2023 Market Size (USD Bn) | 2032 Projected Size (USD Bn) | CAGR (2024-2032) |
| Total Global Market | 60.5 | 89.14 | 4.4% |
| Joint Reconstruction Devices | N/A | Dominant Share | Stable, High-Volume Growth |
| North America Region | N/A | Largest Market Share | Superior Growth Rate |
| Hospitals & ASCs | N/A | Largest End-User Share | Infrastructure Expansion |
What Role Do Robotics, AI, and 3D Printing Play in the Future of Orthopaedic Surgery?
The orthopaedic devices market is undergoing a structural transformation, shifting its focus from hardware production to integrated procedural solutions. The synergy between advanced robotics, Artificial Intelligence (AI) for pre-operative planning, and additive manufacturing (3D printing) is not just improving outcomes—it is fundamentally changing the business model.
Robotic-Assisted Surgery (RAS): RAS platforms, such as Stryker’s Mako and Zimmer Biomet’s ROSA, are moving beyond simple navigation aids to becoming active surgical assistants. These systems allow surgeons to execute precise bone cuts and ligament tensioning with sub-millimeter accuracy, which is critical for implant alignment and long-term joint function. The adoption of RAS is accelerating rapidly, particularly in elective joint replacement procedures, as hospitals leverage the technology to reduce complication rates and attract patient volume.
The AI-Powered Pre-operative Phase: Before the first incision, AI algorithms are now processing vast amounts of patient data, including CT scans and X-rays, to create highly detailed, personalized surgical plans. This level of planning minimizes intra-operative variability, optimizes implant sizing, and reduces procedure time, representing a major advancement in efficiency and patient safety.
3D Printing: Personalized Implants and Orthobiologics: 3D printing is the lynchpin of personalization. It enables the creation of patient-specific instrumentation (PSIs) for surgical guidance, which cuts down on operative time and inventory costs. More significantly, it facilitates the manufacturing of custom porous implants with complex lattice structures designed to mimic human bone tissue. This enhanced porosity promotes faster, more robust osseointegration, leading to reduced rates of revision surgery. Furthermore, 3D printing is accelerating the development of Orthobiologics, which are materials like bone substitutes or growth factors used to accelerate healing and fusion. This segment—though currently smaller—represents a high-opportunity area for personalized biological repair.
These technological advancements collectively address the core clinical needs of the market: minimizing invasiveness, maximizing precision, and accelerating patient recovery times, thereby reducing the overall cost burden on the healthcare system in the long run.
Expert Insight on Industry Evolution
“The 4.4% CAGR is deceptive; it masks the explosive growth occurring in the high-value, digitally-enabled segments of orthopaedics. The industry’s shift is irrevocable: success is no longer defined by the quality of the metallic implant alone, but by the seamless digital ecosystem surrounding it—from pre-operative planning via AI to execution by a robot, and ultimately, follow-up via remote patient monitoring. The race among the 'Big Four' orthopaedic firms—Zimmer Biomet, Stryker, DePuy Synthes, and Smith & Nephew—is now centered on who can create the most integrated surgical platform that encompasses instruments, software, and consumables. Furthermore, the rising use of Ambulatory Surgery Centers (ASCs) for routine joint replacement signifies a critical economic change. ASCs demand devices that support rapid patient turnaround and reduced hospitalization, creating huge demand for MIS-compatible and enhanced recovery (ERAS) protocols. Companies failing to integrate digital and personalized solutions into their core offerings risk rapid obsolescence, regardless of their legacy market position.”
— Dr. Anjali Sharma, Principal Consultant, Medical Devices & Surgical Technologies, Introspective Market Research
A Deep Dive into Regional and Segmentation Dynamics
Regional Leadership: North America’s Innovation Hub
North America is firmly positioned to dominate the Orthopaedic Devices Market throughout the forecast period. The United States, in particular, is the global epicenter for orthopaedic innovation and consumption. This dominance is underpinned by:
- Surgical Volume: Extremely high rates of elective procedures, particularly hip and knee replacements, driven by cultural acceptance of surgery to maintain an active lifestyle into old age.
- Technological Investment: American hospitals and health systems are quick to adopt high-capital equipment like robotic systems, viewing them as essential competitive differentiators. This creates a strong initial market for premium, next-generation devices.
- Favorable Reimbursement: Comprehensive public and private insurance systems ensure that high-cost procedures and innovative devices are widely accessible, accelerating technology diffusion.
- Shift to ASCs: The accelerating trend of joint replacement procedures moving from large hospitals to Ambulatory Surgery Centers (ASCs) in the U.S. is creating a specialized demand for streamlined, efficient, and MIS-focused device kits and inventory systems, a trend less pronounced in other major economies.
While North America leads, the Asia-Pacific (APAC) region is projected to display the fastest rate of long-term growth. This is attributed to massive, underserved patient populations, increasing per capita healthcare spending, and rapidly improving medical infrastructure in countries like China, India, and South Korea, which are beginning to demand the advanced joint reconstruction and trauma solutions that are standard in the West.
Segmentation Analysis: The Joint Reconstruction Imperative
By Type: Joint Reconstruction Devices (JRD) Reign Supreme The Joint Reconstruction Devices segment is the market’s largest and most crucial component. Hip, knee, and extremity replacements are high-volume, high-value procedures, necessary for treating advanced osteoarthritis, the primary driver of the entire market.
- Knee Reconstruction: Driven by the need for enhanced functionality and longer-lasting implants for younger, more active patients. Innovations focus on mobile-bearing inserts and advanced ceramic coatings.
- Hip Reconstruction: The move towards sophisticated bearing surfaces (ceramic-on-ceramic or ceramic-on-polyethylene) and cemented-free fixation systems promotes better long-term outcomes and continues to sustain high market value.
By End User: Hospitals and the Rise of ASCs The Hospitals & ASCs segment holds the largest share. While hospitals remain the central hub for complex spinal and multi-trauma surgeries, Ambulatory Surgery Centers (ASCs) represent the future growth narrative for elective procedures. ASCs offer a lower-cost, high-efficiency setting for total joint replacements. Device manufacturers must now design product lines specifically tailored for the ASC environment—simplified inventory, smaller footprints, and streamlined procedural kits—to capture this burgeoning market segment effectively.
Latest Breakthroughs and Competitive Landscape Shifts
The competitive environment is defined by strategic M&A and relentless pipeline innovation aimed at two key areas: enhanced osseointegration (for lasting fixation) and soft-tissue preservation.
- Targeting Cartilage Repair: Smith+Nephew’s acquisition of CartiHeal (November 2023) for its Agili-C implant, a product designed for the repair of articular cartilage and osteochondral defects, signals a crucial strategic move. This acquisition strengthens Smith+Nephew’s position in the high-growth Sports Medicine and extremities segment, moving the firm closer to providing comprehensive joint repair solutions rather than just replacement. This reflects an industry-wide recognition that addressing earlier-stage joint disease is critical for future market penetration.
- Lower Extremity Precision: DePuy Synthes’ FDA clearance for its TriLEAP lower extremity plating system (October 2023) underscores the increasing specialization within the trauma and extremities market. Modular, anatomically designed plating systems for the foot and ankle address the complex biomechanics of these joints, offering surgeons greater precision and flexibility. Companies like Medtronic plc and Globus Medical continue to push the envelope in the Spinal Devices segment, focusing heavily on intra-operative imaging integration and navigation technologies for complex fusion procedures.
- Vertical Integration: The largest players, including Stryker Corporation and Zimmer Biomet Holdings, are investing heavily in integrating their hardware and software platforms. This vertical approach aims to create a closed-loop system where the patient stays within their ecosystem from diagnosis to rehabilitation, ensuring data continuity and brand loyalty.
Challenges: Navigating High Costs and Regulatory Tides
The primary deterrent to wider market access, particularly in emerging economies, is the High Cost of Orthopaedic Devices and Procedures. A single robotic system can cost millions of dollars, requiring significant upfront capital expenditure from hospitals, which often translates into higher procedure costs for patients. This creates a health equity challenge, limiting access to advanced care for lower-income demographics.
Furthermore, the Regulatory Landscape poses a constant challenge. Novel devices, particularly those involving new materials, biologics, or complex software algorithms (AI/robotics), face protracted and expensive approval processes (e.g., FDA 510(k) or PMA pathways). Maintaining high quality control for complex manufacturing processes, especially 3D printing, adds further operational complexity and cost pressure to manufacturers.
Case Study: The Transformational Power of Personalized, MIS Spine Fusion
A 55-year-old construction foreman in the Midwest U.S. was diagnosed with severe degenerative spondylolisthesis in his lumbar spine, causing chronic, debilitating pain. Conventional open fusion surgery would have required extensive tissue dissection, leading to prolonged hospitalization and a recovery period of six to nine months.
The Advanced Intervention: The patient was treated at a leading orthopedic center utilizing an integrated robotic navigation system (Medtronic’s Mazor X equivalent) and a 3D-printed, porous titanium interbody cage (manufactured by a firm like NuVasive).
- Pre-op Planning: AI software analyzed the patient’s specific spinal curvature and bone density, designing a precise trajectory for pedicle screw placement and determining the optimal size and positioning of the custom-printed titanium cage.
- MIS Procedure: The robot guided the surgeon to execute a Minimally Invasive Surgery (MIS) through tiny incisions. The custom 3D-printed cage—designed with a unique, highly porous lattice—was implanted, ensuring excellent initial stability and maximum surface area for bone growth.
- Outcome: Due to reduced soft tissue damage and blood loss enabled by the robotic MIS approach, the patient was discharged in just two days. The porous titanium implant facilitated rapid bone ingrowth. After only three months, the patient achieved solid fusion and returned to light duty, exceeding traditional recovery benchmarks by over 50%. This successful blend of robotics, MIS, and custom 3D printing demonstrates the clinical superiority and economic benefits that are driving the high-value segment of the orthopaedic market.
About Introspective Market Research
Introspective Market Research is an industry-leading provider of comprehensive market analysis, strategic consulting, and syndicated research reports focused on high-growth sectors, particularly in the global medical device and healthcare industries. We empower global clients to achieve sustainable competitive advantage through meticulous research and predictive modeling.
Call to Action: To obtain a granular view of specific robotic system installations, ASC purchasing strategies, and regional reimbursement trends, secure access to the full report or arrange a private consultation with our principal analysts.
Download Complimentary Sample Report
Contact: Introspective Market Research
Sales Inquiries: sales@introspectivemarketresearch.com
Phone: +1 773 382 1049 (US)