The Global Liquid Biopsy Market has rapidly emerged as one of the most transformative segments within the diagnostics and precision medicine landscape. Liquid biopsy represents a paradigm shift in disease detection and monitoring by enabling non-invasive analysis of biomarkers such as circulating tumor DNA, circulating tumor cells, extracellular vesicles, and other nucleic acids obtained from blood or other bodily fluids.
Unlike traditional tissue biopsies, liquid biopsy offers a safer, faster, and repeatable approach, making it particularly valuable for cancer diagnosis, treatment selection, and real-time disease monitoring. As healthcare systems increasingly prioritize early detection, personalized therapies, and patient-centric diagnostics, liquid biopsy has become a critical tool shaping the future of modern medicine.
Market Overview
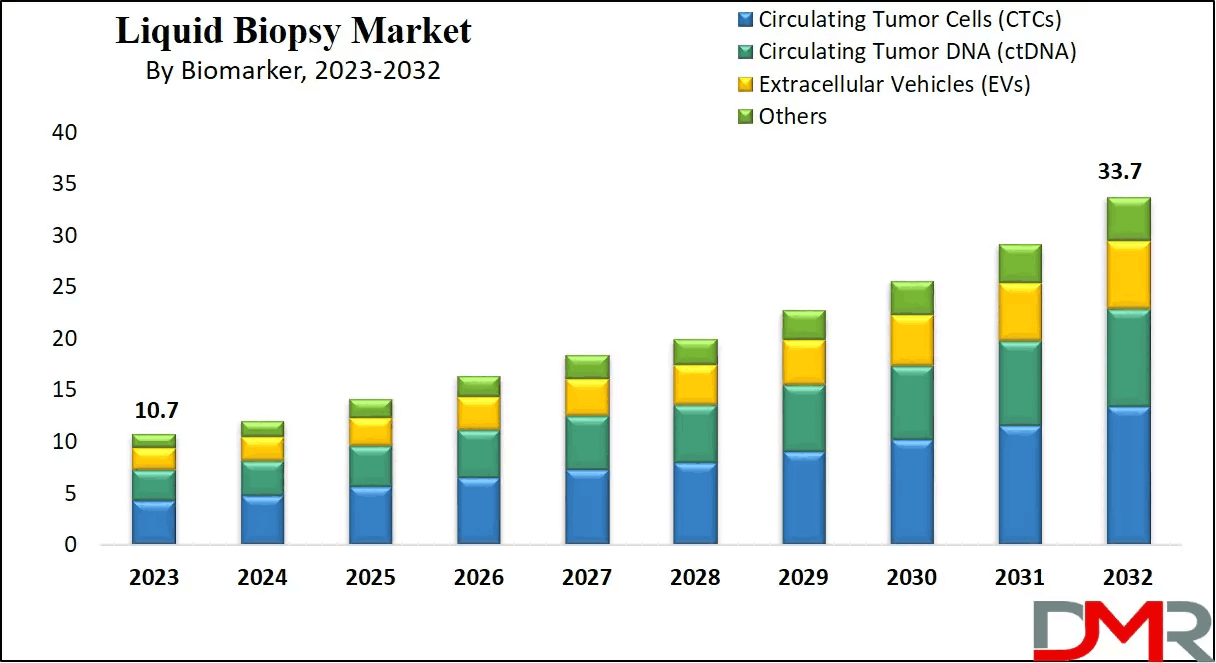
The global liquid biopsy market reached a value of USD 10.7 billion in 2023 and is anticipated to expand substantially to USD 33.7 billion by 2032, registering a compound annual growth rate of 13.6% over the forecast period. This strong growth trajectory reflects increasing adoption of non-invasive diagnostic technologies, rising cancer prevalence, and growing demand for precision oncology solutions. Liquid biopsy has gained widespread acceptance due to its ability to detect molecular changes at an early stage, monitor treatment response, and identify resistance mutations without subjecting patients to invasive surgical procedures.
The market has experienced notable acceleration in recent years, driven by advancements in molecular biology, genomics, and sequencing technologies. Liquid biopsy tests are now increasingly used not only for cancer detection but also for disease prognosis, minimal residual disease monitoring, and therapy optimization. As healthcare providers seek diagnostic tools that deliver faster results with minimal patient discomfort, liquid biopsy is becoming an integral component of clinical decision-making across oncology and other disease areas.
Market Dynamics
The liquid biopsy market is shaped by a combination of clinical needs, technological innovation, and evolving healthcare delivery models. One of the most significant growth drivers is the rising global burden of cancer. Increasing incidence of lung, breast, colorectal, prostate, and other cancers has intensified demand for early and accurate diagnostic tools. Liquid biopsy enables clinicians to detect cancer-related biomarkers even when tumors are inaccessible or too small for traditional biopsy, significantly improving early detection rates.
Another major driver is the growing focus on personalized medicine. Liquid biopsy allows for real-time monitoring of tumor genetics, enabling clinicians to tailor therapies based on individual patient profiles. This capability is particularly important in oncology, where tumor heterogeneity and genetic mutations can influence treatment outcomes. By providing continuous molecular insights, liquid biopsy supports adaptive treatment strategies and improves overall patient outcomes.
Despite its advantages, the market faces certain challenges. High costs associated with advanced sequencing technologies, regulatory complexities, and the need for clinical validation can slow adoption in some regions. Additionally, standardization of testing protocols and interpretation of results remains a critical consideration. However, ongoing research, increasing investment, and improving regulatory clarity are expected to mitigate these challenges over time.
Technology and Biomarker Landscape
Technological innovation is at the core of the liquid biopsy market’s growth. Advances in next-generation sequencing, polymerase chain reaction techniques, and digital PCR have significantly improved the sensitivity and specificity of liquid biopsy tests. These technologies enable detection of low-frequency genetic alterations, making it possible to identify cancer at earlier stages and monitor subtle changes during treatment.
Circulating tumor DNA is one of the most widely used biomarkers in liquid biopsy, providing valuable insights into tumor genetics and mutation profiles. Circulating tumor cells offer complementary information related to tumor biology and metastatic potential. Other biomarkers, including microRNAs, exosomes, and cell-free RNA, are gaining attention as research expands the scope of liquid biopsy applications beyond oncology.
Integration of bioinformatics and artificial intelligence is further enhancing analytical capabilities. Advanced data analytics platforms help interpret complex genomic data, identify clinically relevant mutations, and support evidence-based decision-making. These technological advancements continue to broaden the clinical utility of liquid biopsy and strengthen its role in precision diagnostics.
Application Insights
Oncology remains the primary application area for liquid biopsy, accounting for a significant share of market demand. Liquid biopsy is widely used for early cancer detection, treatment selection, monitoring disease progression, and detecting recurrence. In lung cancer, for example, liquid biopsy plays a crucial role in identifying actionable mutations and guiding targeted therapies.
Beyond oncology, liquid biopsy is gaining traction in other disease areas, including prenatal testing, organ transplant monitoring, and infectious disease diagnostics. In prenatal care, non-invasive prenatal testing using cell-free DNA has become a standard screening method for chromosomal abnormalities. In transplant medicine, liquid biopsy techniques help monitor organ rejection by detecting donor-derived cell-free DNA.
These expanding applications enhance the overall market potential and reduce reliance on a single therapeutic area. As research continues to validate new use cases, liquid biopsy is expected to become a versatile diagnostic platform across multiple medical disciplines.
End-User Analysis
Hospitals and diagnostic laboratories represent the largest end-user segment in the liquid biopsy market. These facilities are increasingly adopting liquid biopsy tests to improve diagnostic accuracy, streamline workflows, and enhance patient care. Academic and research institutions also play a critical role, driving innovation through clinical studies and translational research.
Pharmaceutical and biotechnology companies are another important end-user group, leveraging liquid biopsy in drug development and clinical trials. Liquid biopsy enables efficient patient stratification, real-time monitoring of therapeutic response, and identification of biomarkers for targeted therapies. This integration of diagnostics and therapeutics supports the broader shift toward precision medicine.
Product Type Insights
The liquid biopsy market encompasses a range of products, including kits, reagents, instruments, and services. Consumables such as assay kits and reagents account for a significant share of recurring revenue, driven by routine testing and ongoing clinical use. Instruments, including sequencing platforms and PCR systems, represent a substantial upfront investment but are essential for enabling advanced diagnostic workflows.
Services, including sample processing, data analysis, and interpretation, are gaining importance as healthcare providers seek comprehensive solutions without investing heavily in infrastructure. This trend supports the growth of integrated service-based models within the market.
Regulatory and Clinical Validation Landscape
Regulatory approval and clinical validation are critical factors influencing market growth. Liquid biopsy tests must demonstrate high accuracy, reproducibility, and clinical relevance to gain widespread adoption. Regulatory agencies play a key role in establishing standards that ensure patient safety and diagnostic reliability.
Increasing collaboration between regulatory bodies, healthcare providers, and technology developers is helping streamline approval pathways. As more liquid biopsy tests achieve regulatory clearance, confidence among clinicians and patients is expected to rise, further accelerating market expansion.
Regional Analysis
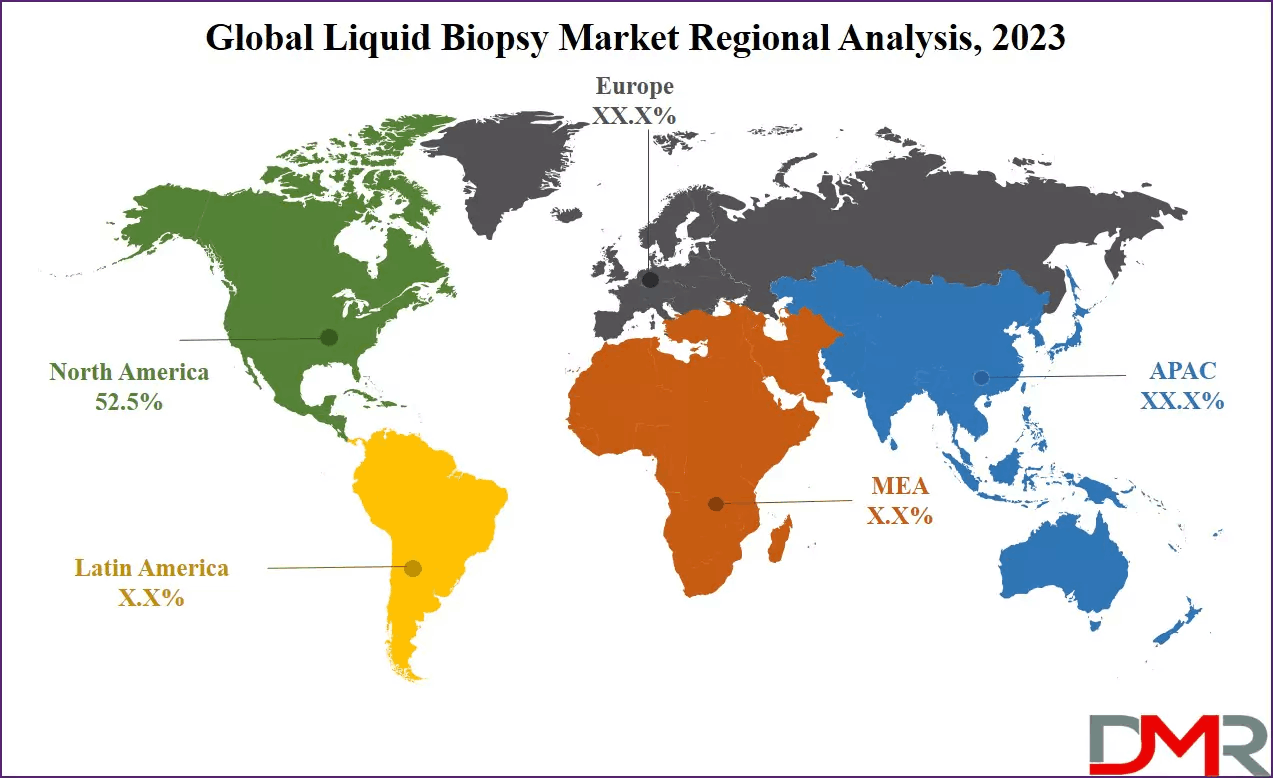
North America dominates the global liquid biopsy market, holding 52.5% of the market share in 2023 and expected to maintain strong growth through 2032. This regional leadership is driven by a well-developed healthcare infrastructure, high healthcare expenditure, and strong investment in research and development. Advanced hospital systems and diagnostic laboratories provide an ideal environment for the adoption of innovative diagnostic technologies such as liquid biopsy.
The region is also home to a robust ecosystem of biotechnology and pharmaceutical companies, along with leading academic and research institutions. These organizations actively invest in the development of highly precise and effective liquid biopsy tests, supporting continuous innovation. Favorable reimbursement policies, early adoption of precision medicine, and strong clinical research activity further reinforce North America’s dominance in the market.
Europe represents another significant market, characterized by strong research capabilities and growing adoption of personalized medicine. Asia Pacific is expected to witness rapid growth due to increasing cancer incidence, improving healthcare infrastructure, and rising awareness of advanced diagnostic solutions. Emerging markets across Latin America and the Middle East & Africa present long-term opportunities as access to modern healthcare expands.
Download a Complimentary PDF Sample Report: https://dimensionmarketresearch.com/report/liquid-biopsy-market/request-sample/
Competitive Landscape
The liquid biopsy market is highly competitive and innovation-driven, with participants focusing on technological differentiation, clinical validation, and strategic collaborations. Companies are investing heavily in research and development to expand test menus, improve sensitivity, and reduce costs. Partnerships between diagnostic developers, healthcare providers, and pharmaceutical companies are becoming increasingly common, supporting integrated diagnostic and therapeutic solutions.
Competition is also influenced by intellectual property portfolios, regulatory approvals, and global distribution capabilities. As the market matures, consolidation and strategic alliances are expected to play a greater role in shaping the competitive landscape.
Impact of Precision Medicine and Digital Health
The rise of precision medicine and digital health initiatives is significantly influencing the liquid biopsy market. Integration of genomic data with electronic health records and clinical decision-support systems enhances the utility of liquid biopsy results. Digital platforms enable seamless data sharing, longitudinal patient monitoring, and real-time analytics, supporting personalized care pathways.
As healthcare systems adopt value-based care models, diagnostic tools that improve outcomes and reduce costs are increasingly prioritized. Liquid biopsy aligns well with these objectives by enabling early intervention, reducing invasive procedures, and optimizing treatment strategies.
Future Outlook
The future outlook for the liquid biopsy market remains highly promising, supported by continuous technological advancements, expanding clinical applications, and increasing acceptance among healthcare professionals. As costs decline and testing becomes more standardized, liquid biopsy is expected to transition from a specialized diagnostic tool to a routine component of clinical practice.
Emerging applications in early cancer screening, minimal residual disease detection, and multi-cancer testing represent significant growth opportunities. Ongoing research into novel biomarkers and analytical methods will further enhance diagnostic accuracy and expand the scope of liquid biopsy. As precision medicine continues to evolve, liquid biopsy is poised to play a central role in shaping the future of diagnostics and personalized healthcare.
Frequently Asked Questions
What is a liquid biopsy?
A liquid biopsy is a non-invasive diagnostic method that analyzes biomarkers such as circulating tumor DNA or circulating tumor cells from blood or other bodily fluids.
Why is liquid biopsy important in cancer care?
It enables early detection, treatment monitoring, and identification of genetic mutations without the need for invasive tissue biopsies.
Which region leads the liquid biopsy market?
North America leads the market due to advanced healthcare infrastructure, strong research activity, and high adoption of precision medicine.
What technologies are used in liquid biopsy testing?
Key technologies include next-generation sequencing, polymerase chain reaction, digital PCR, and advanced bioinformatics platforms.
What is the long-term outlook for the market?
The market is expected to grow steadily through 2032, driven by expanding applications, technological innovation, and rising demand for non-invasive diagnostics.
Summary of Key Insights
The liquid biopsy market is transforming the global diagnostics landscape by offering non-invasive, precise, and patient-friendly testing solutions. Strong market growth is driven by rising cancer prevalence, advancements in molecular diagnostics, and increasing adoption of personalized medicine. North America dominates the market, supported by robust healthcare infrastructure and research investment, while other regions offer significant growth potential. As clinical validation expands and costs decline, liquid biopsy is set to become an essential component of modern healthcare, enabling earlier detection, better treatment outcomes, and more efficient disease management.
Purchase the report for comprehensive details: https://dimensionmarketresearch.com/checkout/liquid-biopsy-market/

