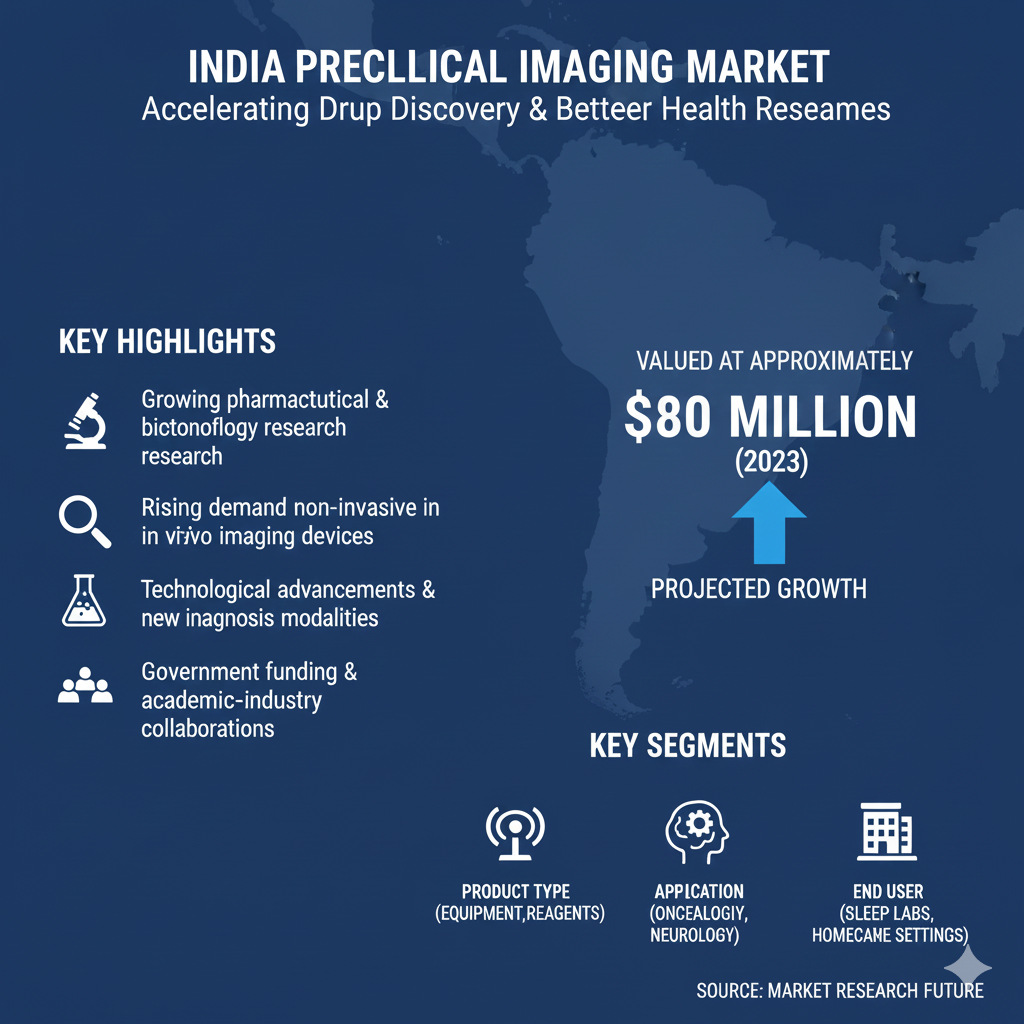
Looking ahead, India’s preclinical imaging sector is expected to play an even larger role in global R&D chains as AI-enhanced workflows and multi-site standardization expand. Automated pipelines that ingest, process, and analyze imaging data will help labs handle larger study volumes with improved consistency. These technology trends are central to optimistic India Preclinical Imaging Market Projections that anticipate continued investment and capacity growth.
Standardized imaging protocols across multiple Indian sites will make it easier for sponsors to compare results and combine datasets from different locations. This harmonization is particularly valuable for large programs requiring replication, multiple disease models, or cross-validation. As more institutions participate in such standardized networks, India will be able to support increasingly complex and distributed research designs.
Finally, deeper integration between preclinical imaging programs and global clinical trial strategies will solidify India’s role as a key node in pharmaceutical innovation. By using imaging biomarkers that translate from animal models to human studies, sponsors can design more efficient, data-driven development pathways. This combination of technology, talent, and strategic integration underpins strong long-term growth expectations for India’s preclinical imaging market.
FAQs
Q1. How will AI shape future preclinical imaging in India?
AI will streamline analysis, reduce turnaround times, and improve the predictive value of imaging datasets.
Q2. Why is standardization across sites so important?
It ensures data comparability and reliability, which is crucial for supporting large, multi-center research programs.